Modern regulatory information management for medtech
A single, cloud platform for end-to-end regulatory process digitization and automation—designed by and for regulatory affairs professionals
Request a demo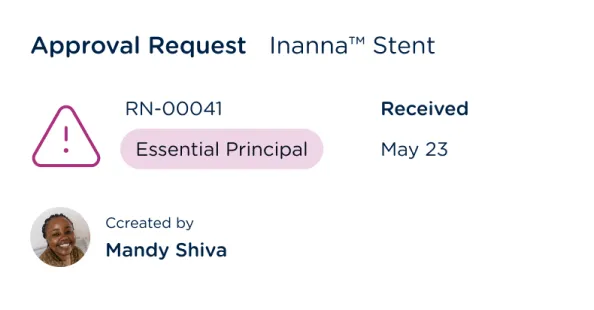



Platform features
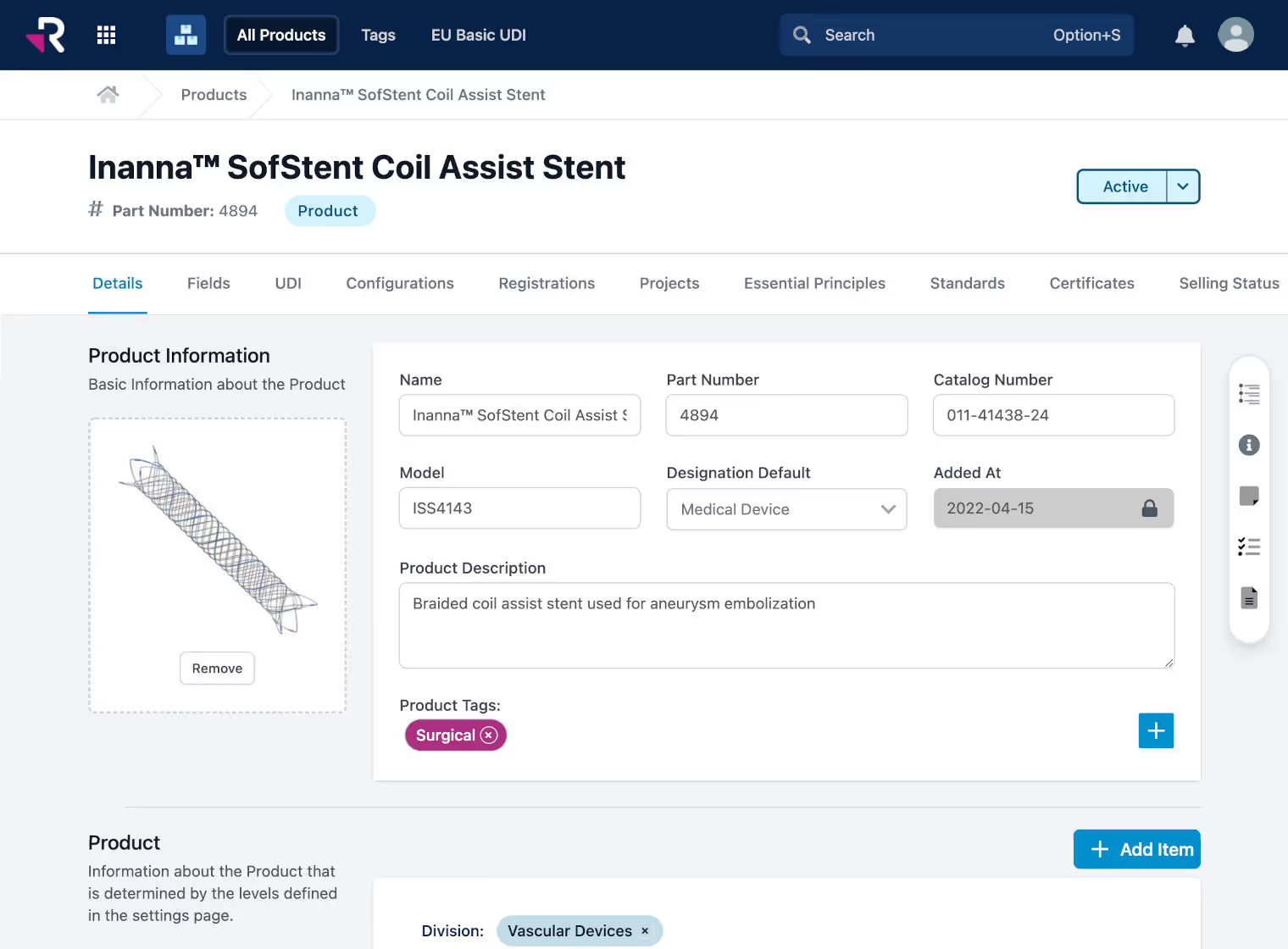
Manage regulatory projects and resources more effectively, get new products to market more quickly, and reduce revenue risk of non-compliance issues, product recalls, and unexpected expirations.











Omron reduces time spent on regulatory reporting by 98%
Discover how Rimsys helped Omron, a leading global medical device manufacturer of heart monitoring equipment, blood pressure monitors, digital thermometers, and nebulizers, digitize product registrations, eliminate information silos, and streamline reporting across office locations.

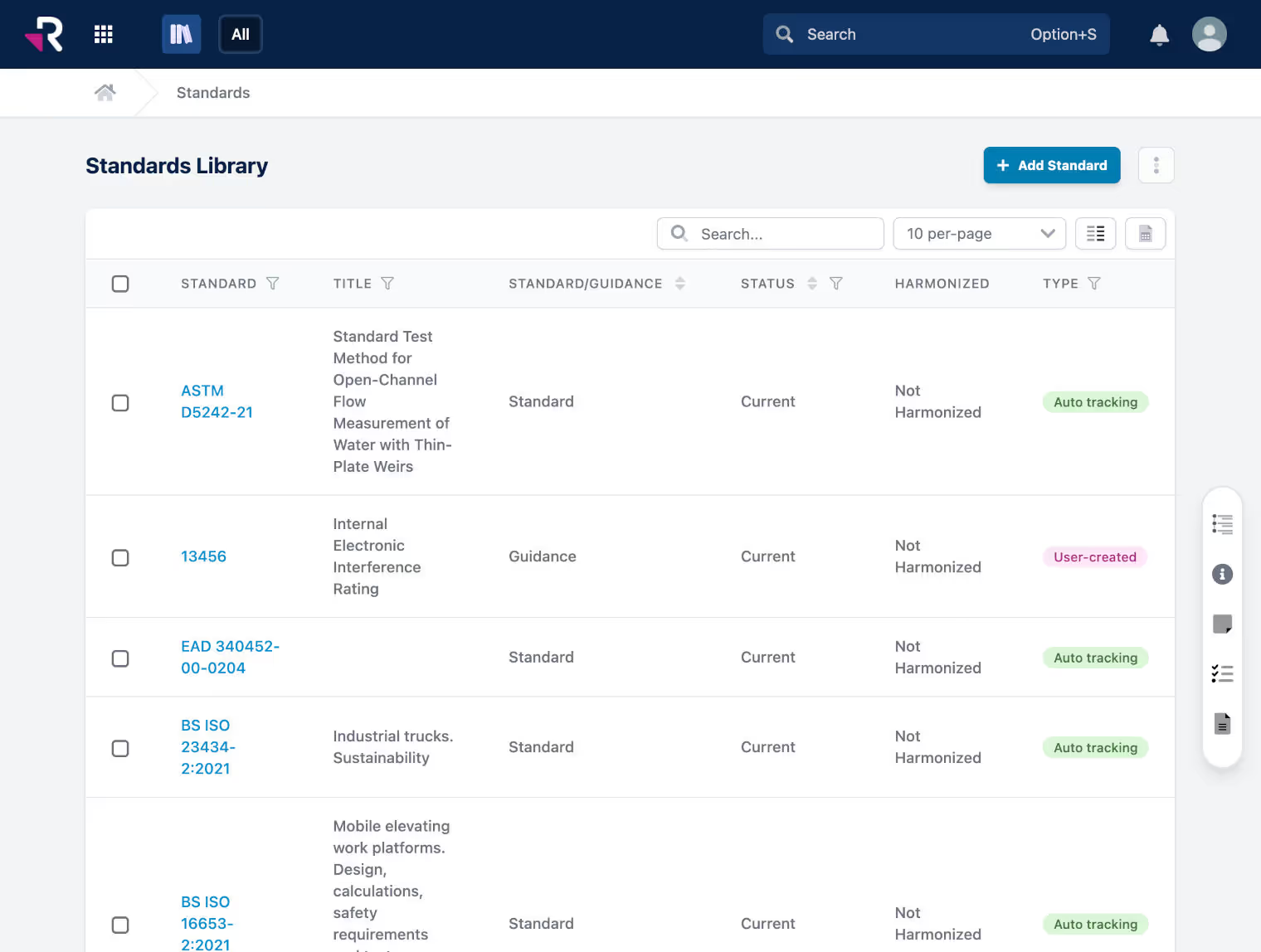
All of your RA processes in one integrated platform

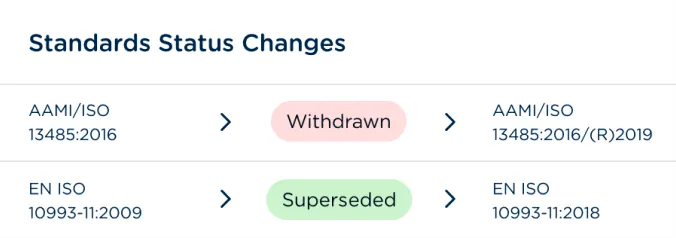
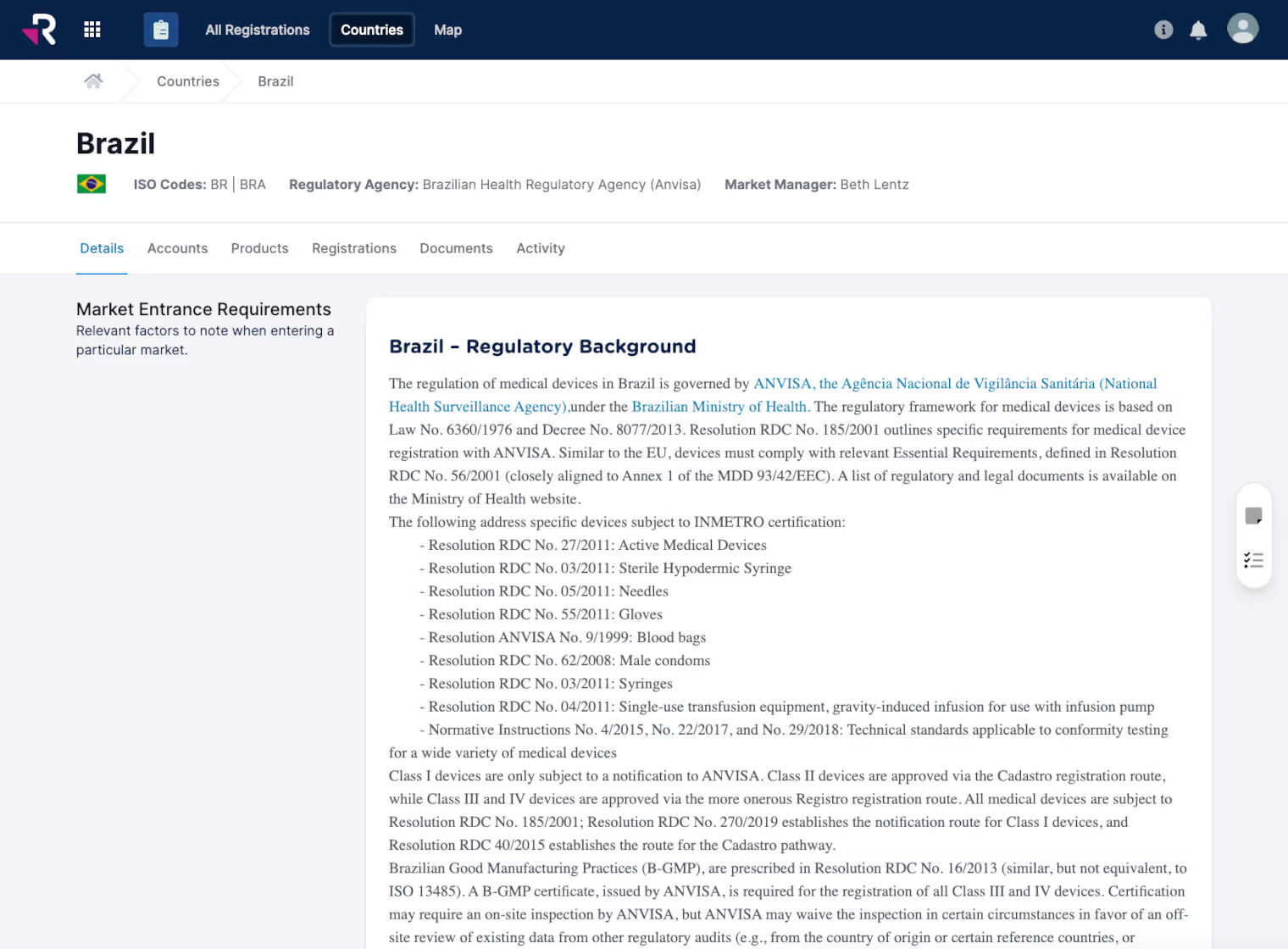
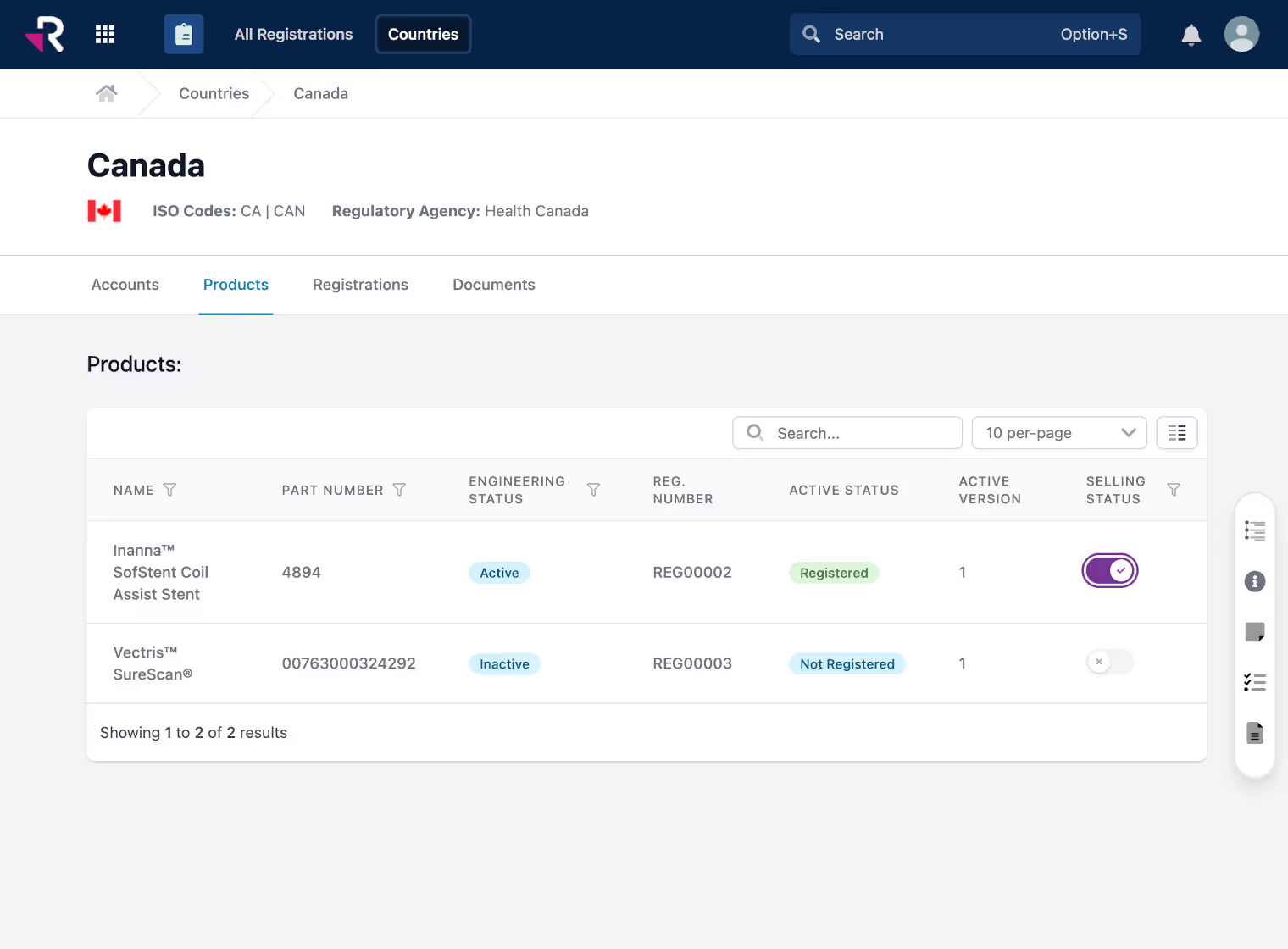
Monitor and manage global product registrations, certificates, and expirations


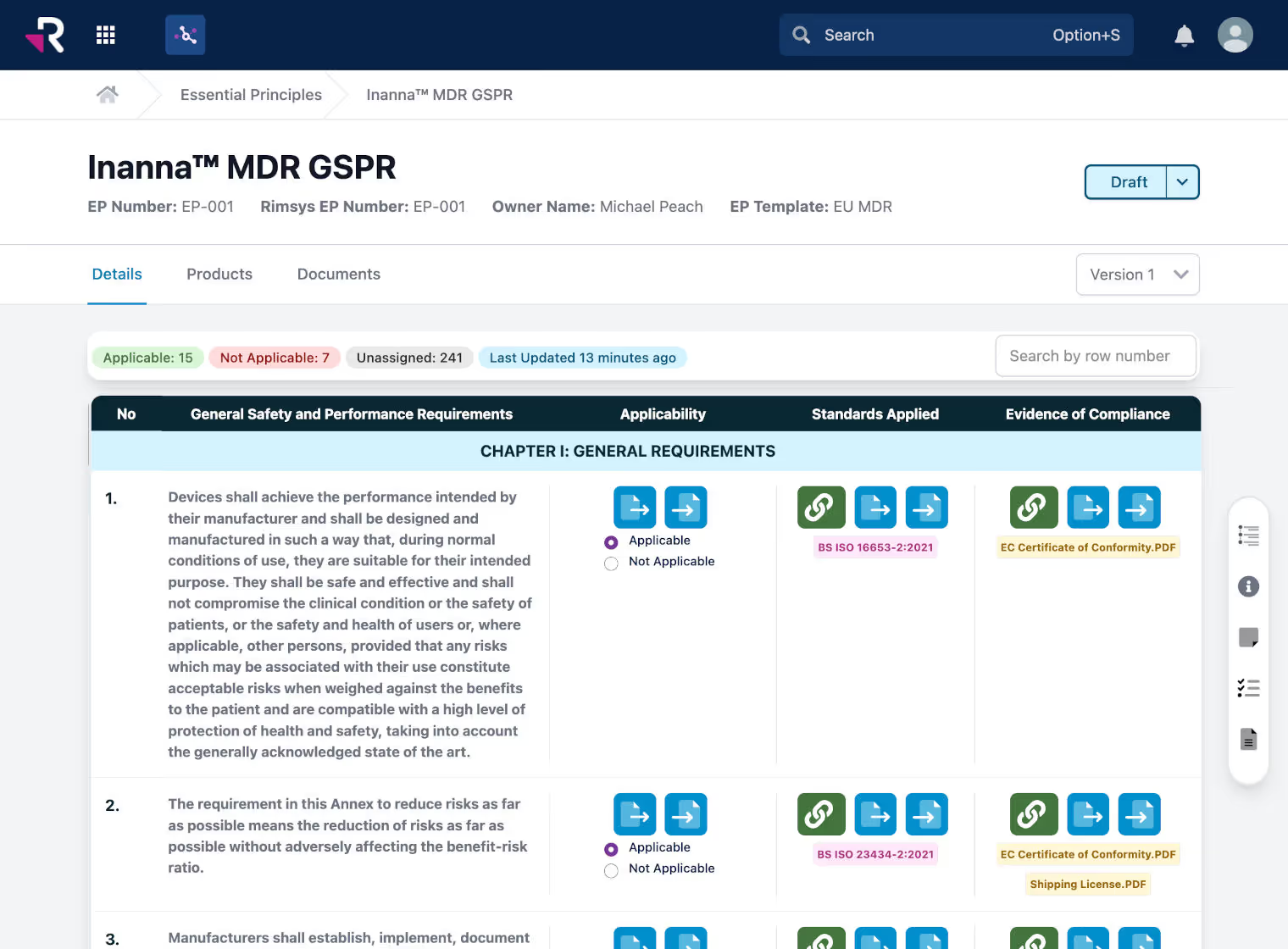
Create and maintain essential principles/general safety and performance requirements (GSPR) tables


Harness the power of Rimsys AI, a suite of intelligent AI Agents designed exclusively for MedTech







