Streamline global submissions
Collaboratively author, manage, approve, and publish regulatory submissions
Request a demo
Getting new medical devices to market requires regulatory affairs teams to properly decipher entrance requirements and coordinate the assembly of complex submission dossiers for each country. Manually managing these processes over email and spreadsheets only increases the challenge.
Rimsys simplifies global submission management with access to quality regulatory intelligence, and intelligent tools that provide complete control over submission authoring, assembly, approvals, and publishing.
Medical device regulatory submission software from Rimsys

Access information and government-specific templates for over a dozen different countries
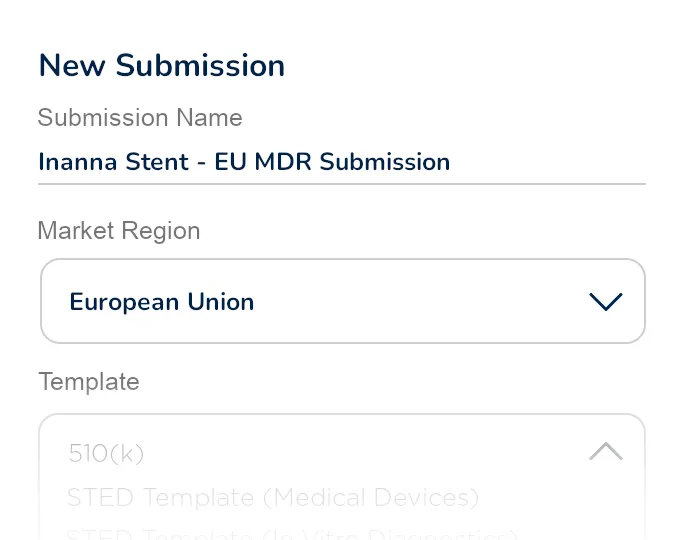
Build submission plans using fully customizable templates for FDA 510(k), STED, and other common market applications
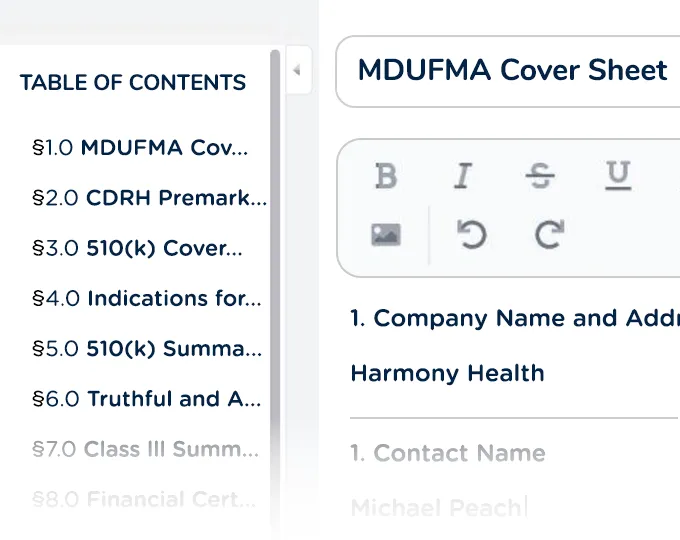
Create, edit, and share structured submission content without external documents or spreadsheets
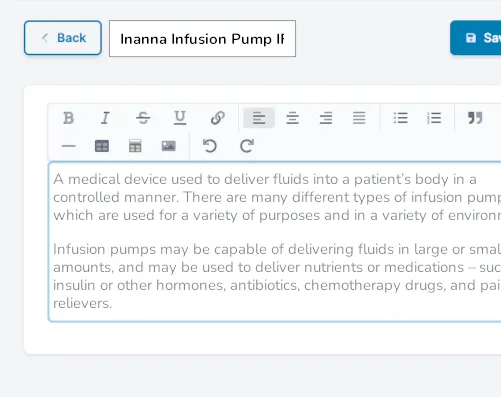
Create variables, snippets, forms, and tables for easy and consistent reuse across submissions
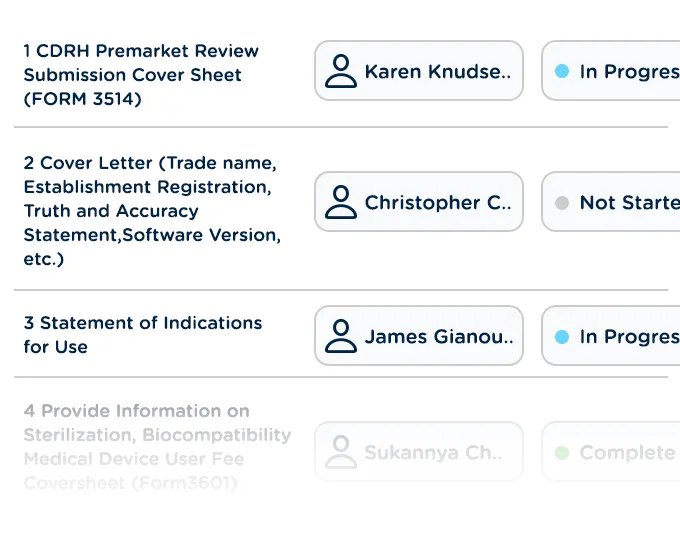
Assign tasks, track correspondence with health authorities, and manage approval processes

Render documents in the correct PDF format with auto-generated TOCs and appendices based on your content plan
FDA medical device registration process - getting new products to market in the U.S.
An overview of the FDA medical device registration process, including how to classify a device and which submission process to use. This article covers the 510(k), PMA, and De Novo submission processes, and provides links to resources with additional information.




