Rimsys Gets You to EUDAMED Compliance Faster
Collect your UDI data and get your regulatory ducks in a row — and get started today with our free spreadsheet template.
Get your free spreadsheet template
Why EUDAMED Compliance Is So Challenging
We know the challenges MedTech firms are facing:
As of March, only 2 companies had posted data to EUDAMED
Only 3 training sessions for EUDAMED compliance with limited capacity
UDI data is scattered, incomplete, or missing required details
Each country has unique regulatory databases, creating inconsistencies
Access point configuration is complex, takes over 3 months, and often fails
XML and DTX formats are major barriers to bulk data submission
Here are a few quotes from our clients. Can you relate?
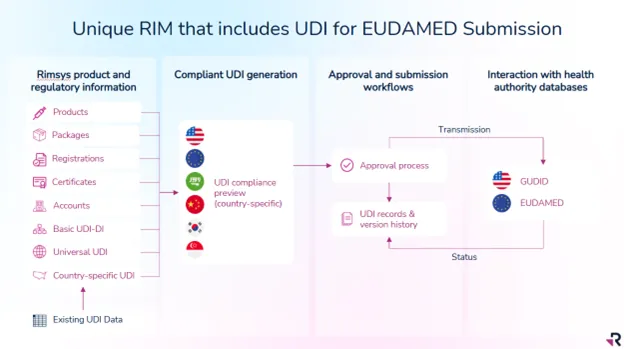
The Only Unified RIM System for EUDAMED Compliance
Rimsys is the only provider that delivers EUDAMED compliance within a full Regulatory Information Management (RIM) system, giving you a unified approach to managing regulatory data across products, submissions, and markets.
Here’s how we help:
• Proven experience with Top 25 MedTech companies
• Universal UDI® that uses common attributes across all markets to simplify data collection and maintenance
• Automated data preparation, verification, and submission
• Scalable and future-proof to support global expansion and technical maintenance
Purpose-Built Tools and Expert Guidance
With Rimsys, you don’t have to navigate EUDAMED alone:
- Use our free spreadsheet template to collect and structure UDI data
- Automated verification and transformation workflows
- Pre-configured submission process requiring minimal input
- Access to internal experts who are members of the MedTech Europe UDI and EUDAMED working groups
- Benefit from unified data management across all your regulatory activities
- Visit our EUDAMED Resource Center to learn the 4 Steps to Get EUDAMED Ready.
What It Takes to Post Data

Collect and structure UDI data (start with our free spreadsheet template)

Create a EUDAMED playground account

Set up M2M transmission (typically takes 3 months)

Complete XML data validation (typically takes 3 months)
What Sets Rimsys Apart
Rimsys is the only provider that delivers EUDAMED compliance within afull Regulatory Information Management (RIM) system, giving you a unifiedapproach to managing regulatory data across products, submissions, and markets. Here's how we help:
• We are proven. Top 25 MedTech companies have chosen us for product registration and EUDAMED compliance
• We are the only RIM provider with Universal UDI® which uses common attributes across markets for simplified ongoing technical maintenance and compliance
• Rimsys has a working Access Point and connection to the EUDAMED system and is actively posting Basic UDI and UDI data, removing common obstacles to compliance progress
• Our experts are members of the MedTech UDI and EUDAMED working groups and have deep knowledge of the data requirements and regulation
• Rimsys fully manages electronic data transmission, minimizing IT impact and support needed to establish and maintain connection to a growing number of regulatory databases
• Our purpose-built MedTech RIM platform is ISO 27001, SOC II Type 2, GDPR, and FDA 21 CFR Part 11 compliant and audited regularly