
The EU Commission has recently announced updates for completing and implementing EUDAMED based on amendment 2024/1860. This article outlines the current EUDAMED timelines and our point of view on these timelines to help industry prepare accordingly.
Current EUDAMED Timelines:
- The target date for the first mandatory application of functional EUDAMED modules is still January 1, 2026. The Vigilance module is expected to be mandatory beginning in Q3 2026 with full EUDAMED functionality planned for Q2 2027.
- The Actor, UDI & Devices, Certificates, and Market Surveillance modules are currently under audit. The independent Minimum Viable Product (MVP) audit is intended to assess and confirm functionality and interconnectivity of the modules that are deemed audit ready. This audit is foreseen to be completed by Q2 2025.
- Mandatory use of each module is to commence six (6) months after the module is declared fully functional through the independent audit and publication in the Official Journal of the European Union (OJEU). The Actor, UDI & Devices, Certificates, and Market Surveillance modules are expected to be declared fully functional at the end of Q2 2025, leading to their mandatory application date of January 1, 2026.
- The Actor, UDI & Devices, Certificates, and Market Surveillance modules are expected to be declared fully functional by the end of Q2 2025 and mandatory for industry use on January 1, 2026.
- The Vigilance module is not part of the ongoing MVP audit and will not be declared fully functional along with the previously mentioned modules. The revised timeline indicates that the audit of that module will occur between Q2 and Q3 of 2025, with the goal of the mandatory application date in Q2 of 2026.
- The development of the Clinical Investigation/Performance Studies (“CI/PS”) module is intended to continue through Q3 2026. An audit to assess the CI/PS module together with the other five (5) modules will be completed once the CI/PS MVP has been developed.
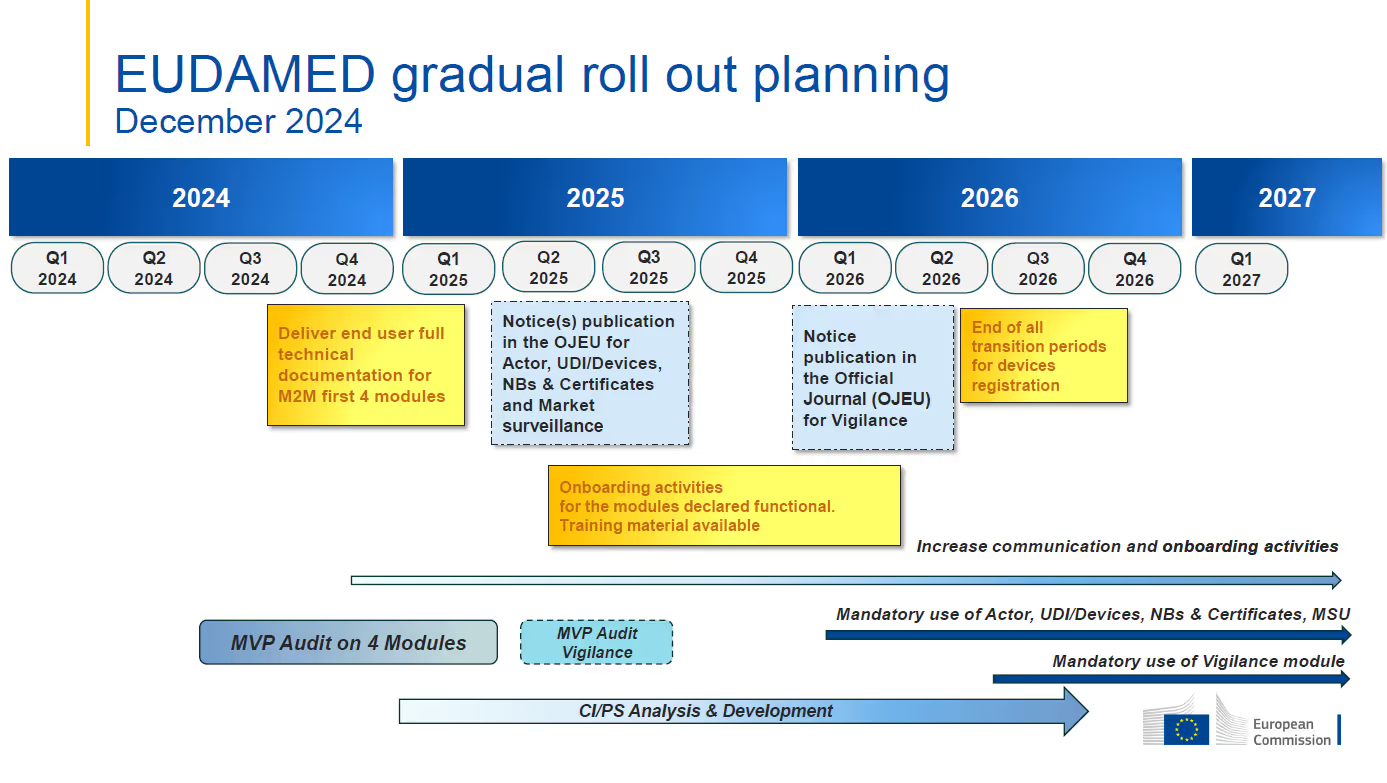
Photo courtesy of the European Commission
Here is how Rimsys views the impact of this announcement for each stakeholder group:
Rimsys
UDI is front of mind as well as future interaction with the Vigilance module. There is no change to our current plans, as Rimsys will continue to develop UDI and Post Market Surveillance functionality regardless of the updated target dates. We also recognize the potential impact of establishing data transfer (DTX) capabilities to interact with EUDAMED in a machine-to-machine (M2M) capacity. With the publication of the final requirements needed for M2M DTX to EUDAMED, Rimsys is positioned to finalize our connection and deliver M2M capabilities as part of the EUDAMED solution.
Industry/customer
Since the European Commission(EC) has made multiple updates to EUDAMED timelines, we expect industry will have some reluctance to accept the new target dates. As a result, this could delay re-engagement with EUDAMED preparations. However, we do not expect the EC to push these updated timelines. Manufacturers that don’t have a plan to submit data to EUDAMED by Q2 this year should expect significant challenges to meet these deadlines. With the audit of expected modules underway with the associated technical documentation published, Rimsys recommends taking steps to organize regulatory data now and submit their information early to all available EUDAMED modules.
EU Commission
The EC strongly recommends industry continues to establish its solution and to submit data on a voluntary basis. Do not wait. The commission’s position is that submitting data early will give companies an advantage by having their data “in” before the onslaught of the entire global MedTech industry, all trying to add data at the same time EUDAMED becomes mandatory. These companies will also be in front of the line to work with the commission resources if data submission issues occur.
* Note - this article includes regulatory interpretations and opinions from the Rimsys team. We try to be as informative as possible, but this information isn’t intended to serve as a substitute for official guidance from regulatory authorities.
Similar posts







.avif)


