Free, community-driven global regulatory intelligence
Rimsys is empowering the medtech community with a free, centralized hub for global regulatory intelligence data. Quickly find the information you need backed by our powerful search capabilities to make more informed decisions about market access for your products and execute faster.
Join the beta waitlist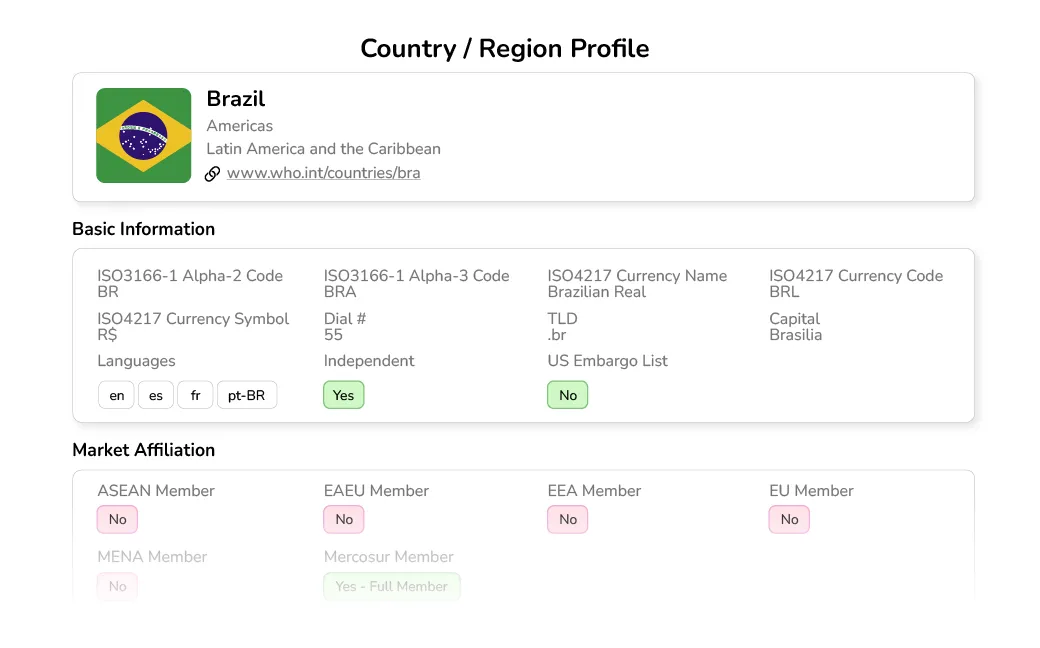

As part of our mission to improve the availability of life-changing medical technologies, it's our belief that generally available regulatory intelligence information should be easy to find and accessible to everyone.

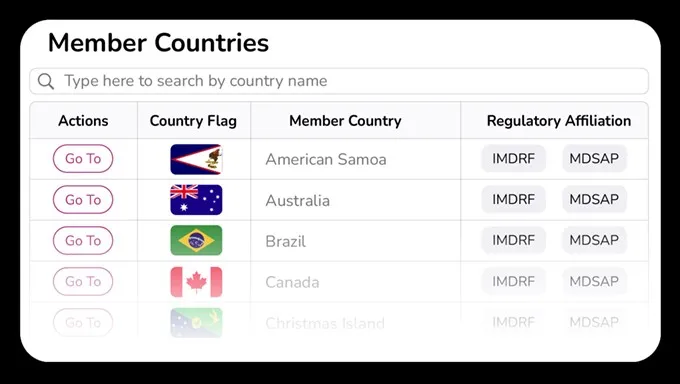
Search and drill down into specific countries, regions, and regulatory affiliations to quickly find intelligence data for all regulated markets (and quickly see those that aren't).
Easily find legislation details for all regulated countries along with their applicable UDI requirements
Find risk classifications and definitions for medical devices and IVDs by market and requirements for each, including submission types, application timelines, fees, and more.
Understand varying market entrance requirements and documentation needed for each country by risk class.
Empower your peers with knowledge by submitting a change request when you see a necessary update. RAPS RAC holders can even qualify for recertification credits (more details below).
Regulatory intelligence, revolutionalized

Find global regulatory requirements in one place. No more consolidating information from countless searches or trying to translate critical documents.


Its part of our core values to empower each other, and we want to enable the medech RA community to empower each other with their knowledge to keep Rimsys Intel high quality and accurate.


Great, community-sourced regulatory intelligence requires great minds, so we're collaborating with RAPS to allow RAC holders to qualify for recertification credits by helping to keep Rimsys Intel up to date.