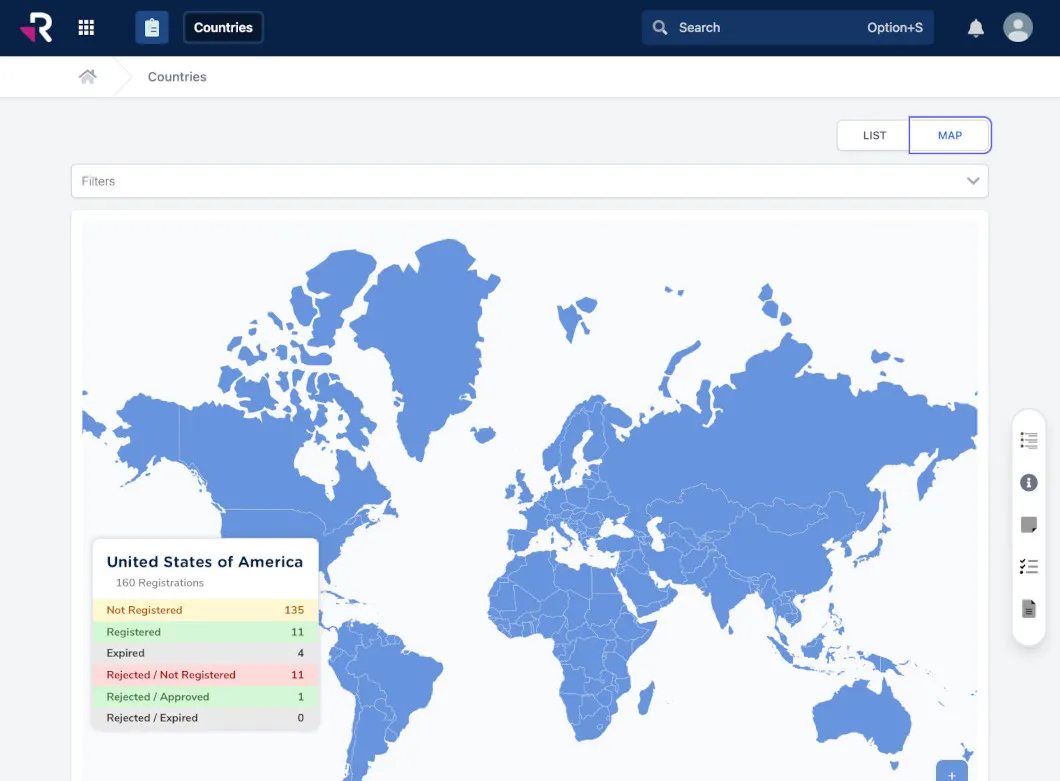
Get instant visibility into global registrations and selling status
No magic magnifying glass will actually show you where your products are sold, but Rimsys can help. You shouldn’t have to spend 50% of your time looking for information. Our platform centralizes regulatory data and automates manual tasks to unlock your productivity.
Learn more
Learn more

Download our RIM benefits data sheet to see what your team could gain from digitizing and automating regulatory activities.
Get data sheetSee it for yourself
Schedule a free custom demo with our regulatory experts. We’ll discuss your specific needs and walk you through the key features of our platform.
Get a demoOmron reduces time spent on regulatory reporting by 98%
Discover how Rimsys helped Omron, a leading global medical device manufacturer of heart monitoring equipment, blood pressure monitors, digital thermometers, and nebulizers, digitize product registrations, eliminate information silos, and streamline reporting across office locations.


Centralize all regulatory information
Create, organize, and consolidate a “single source of truth” for all documents and information. Ensure that everyone across your organization is working with consistent, up-to-date information.
Automate regulatory processes
Streamline submissions with entrance requirements for over 100 countries, government-specific templates, approval workflows, and digital authoring and publishing. Monitor expirations, standards, and regulatory changes to ensure products can stay on the market without disruption.

A complete product-centric platform for all regulatory activities

Monitor and manage global product registrations, certificates, and expirations


Create and maintain essential principles/general safety and performance requirements (GSPR) tables







