FDA listed, cleared, approved, granted - what IS the difference?

The terms “listed," "cleared,” “approved,” and “granted” all refer to a finding or status from the FDA that authorizes a medical device to be legally placed on the market (for sale) in the United States. As a result, these terms tend to be used interchangeably, but they definitely don’t mean the same thing. Each references a unique pathway to market that is based on the device’s risk class. This article explains the differences between each term and what level of FDA review they require.
Market pathways depend on device classification
A business that is involved in the production and distribution of medical or in vitro diagnostic devices (intended for distribution and use in the United States) is required to register its establishment annually with FDA, using a process called establishment registration. This process requires putting information into an FDA database on their website. This also requires the business to list the devices and the activities performed on those devices, at their establishment. But before you can do this, you need to identify the proper classification of the device(s).
The FDA uses three levels of classifications for medical devices - each carrying a different patient risk value. Once the correct classification is determined, you must then choose the proper registration pathway – Premarket Notification (otherwise known as 510(k)), Pre-Market Approval (PMA), or De Novo process. Before you can legally market your device in the US, it must be FDA Cleared or Approved or in the case of the De Novo process, Granted.
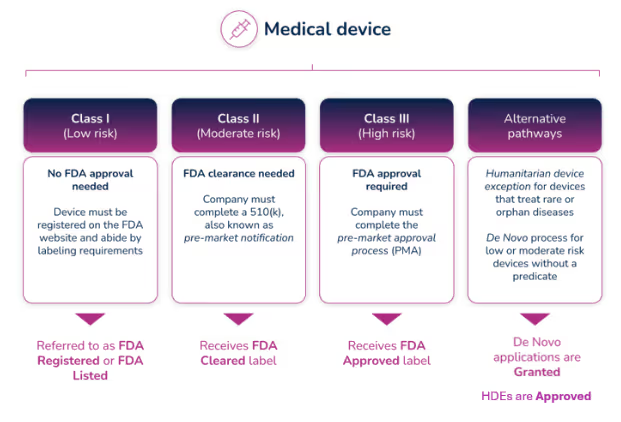
What do the different FDA terms mean?
Regulatory professionals hear the terms Registered, Cleared, Approved, and Granted throughout the medical device industry, and even they are sometimes confused about the differences between them. However, the distinctions are significant, and it’s important to understand those differences and how and when to use them.
- Registered/Listed: A company that has registered with the FDA and has listed their device and the activities performed on those devices at that establishment, into the FDA's registration and listing database. It applies to all class devices, but for most of the Class I devices, it is the only form of registration with FDA. Important to know: the FDA does not issue any type of device registration certificates to medical device facilities.
- Cleared/Clearance: Most of the Class II and some Class I devices require a Pre-Market Notification (510(k)) submission. Before you can sell a device to the public, each submitter must receive an order, in the form of a letter, from FDA which finds the device to be substantially equivalent (SE) and states that the device can be sold in the U.S. This order clears the device for commercial distribution.
- Approved/Approval: A premarket approval (PMA) is the hardest type of device marketing application required by FDA for class III medical devices. To be legally sold on the market, they must undergo an extensive review and approval process. Following a successful submission of a (PMA) or a Humanitarian Device Exemption (HDE), the device is given Approval by FDA.
- Granted: Medical devices using the De Novo process will be Granted approval by FDA before they can be legally marketed in the United States.
Most Class I and some Class II medical devices are exempt from 510k submission requirements.
All other Class II devices require 510(K) clearance as a premarket submission to FDA to demonstrate that the device is safe and effective. Clearance is based on the device being substantially equivalent to an existing, legally marketed device, that does not require premarket approval (PMA). Medical devices in the 510(k) category receive an FDA clearance to bring the device to market.
All Class III devices require a Pre-Market Approval (PMA) - the most stringent type of device marketing application required by FDA. Premarket approval is the required process of scientific review to ensure the safety and effectiveness of Class III devices. Medical devices in this category receive FDA approval to bring the device to market.
Novel devices that don’t have a predicate on the market are classified as Class III by default. However, companies can use the De Novo process to request that the FDA review the risk and safety information of the device for possible re-classification. When a De Novo request is granted, the device is re-classified as Class II, and the device may be brought to market.
Companies can also submit a Humanitarian Device Exemption (HDE) application for Class III devices. A Humanitarian Use Device (HUD) is a device that is intended to benefit patients by treating or diagnosing a disease or condition that affects fewer than 4,000 individuals in the United States per year. The HDE application is like a PMA application, but it is exempt from the effectiveness requirements of a typical PMA.
A relatively newer term being used now is the Emergency Use Authorization (EUA). This is when the Secretary of Health and Human Services declares that there may be circumstances justifying the authorization of emergency use of medical devices, such as during the COVID-19 pandemic. The FDA may issue an EUA to authorize unapproved medical products (or unapproved uses of approved medical products) so that they can be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions when certain criteria are met.
Checking the status of a device with the FDA
The FDA provides several ways to check if devices are approved, cleared, or granted.
To search for FDA-approved or FDA-cleared products by device name or company name:
- Go to the Devices@FDA Database.
To search for FDA-granted products by device name or company name:
- Go to the Device Classification Under Section 513(f)(2)(De Novo) database.
To search for FDA Emergency Use Authorization devices, go to the listing here.
Conclusion
Terminology is only one of the things that can be confusing about the FDA’s processes. Using the wrong terminology can impact your company’s reputation, and possibly have some legal implications, but more importantly, it can mean that you don’t have a clear understanding of how to bring your product to market.
Making sense of the different FDA processes can be challenging—especially for companies that are bringing devices to the market for the first time. For a detailed walkthrough of the steps, documents, and timeline associated with each path to market, see our Beginners Guide to the 510(k), Beginner’s Guide to the FDA PMA Submission Process, and Beginner’s Guide to the FDA De Novo Process.
Similar posts










